I wish you contribute articles from Asian nations and Oceania.
e-mail:info_web@umelab.net
TEL: 0561-62-3311
(when calling from overseas: +81-561-62-3311)
Contact: umezawa@aichi-med-u.ac.jp
Umezawa Lab focuses on finding chemical substances that could potentially be used as medical drugs for cancer, and immunological diseases. We look for active chemicals and those precursors from the various resources in nature, including soil microbial metabolites, and derivatives of tropical plants. We call this process “mono-tori”, as literally in Japanese “mono” refers to an active substance, and “tori” refers to catching or trapping. (In more general words, it is called simply “screening”.)
Once we find reactive fractions in the screening assays, we proceed to distinguish the reactive substance by taking advantage of the various extraction methods, and finally delineate a structure of the substance.

We also do research on molecular designing to modify natural product structurally.
develop our understanding of cellular signal pathways in diseases using our intracellular signal inhibitors. Additionally, signal inhibitors can be developed into new medical drugs that work through a unique mechanism. As this could directly benefit for the social welfare, we also do research to develop them into medical drugs.
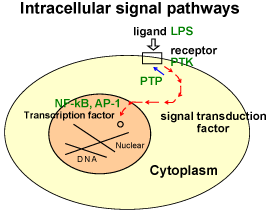
Diseases can be now explained by molecular mechanism. Many ongoing studies in the field of biology focuses on their research in molecular levels. Malignant diseases are gradually recognized as a result of abnormalities in intracellular signal pathways. In an effort of finding better treatment of such diseases based on this notion, a concept of approach to treatment, referred as “molecular targeting therapy” has been developed. Molecular targeting therapy involves an inhibition of the specific abnormal signal pathway and so it is expected to bring about better treatment with lesser side effect.
It is gradually recognized that specific abnormal intracellular signal pathways explain not only cancer but also diabetes and inflammatory diseases such as rheumatism and arteriosclerosis. We therefore screen chemical substances from the natural resources that could play an important role in the development of those diseases. If the signal pathway indeed plays an essential role in a certain disease, inhibiting it by a chemical substance from our screening assay will be a good candidate for molecular targeting therapy.
What the most important to find useful chemicals in molecular targeting therapy is to focus on a signal pathway that certainly involves malignant progression of diseases. Actually, researchers in molecular biology may not be in the best position in selecting the screening targets. Rather, doctors in clinical practice would have a better insight in the important signals in diseases. Therefore, collaboration with them is extremely important for us.

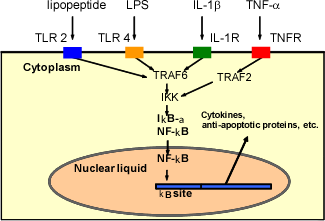
NF-κB is a protein that functions in the nucleus, leading to various diseases. Its well-known function is as a transcription factor, which binds the genomic DNA in the nucleus to produce the specific set of proteins. The NF-κB producing proteins normally work to protect the own cells by strengthening immune responses from invaders and by avoiding an unwanted cell death. However, excess activation of NF-κB can lead to many diseases including cancer and rheumatism.
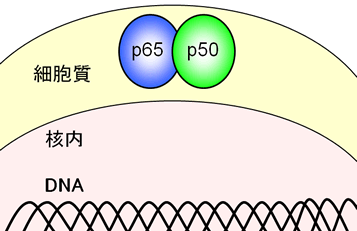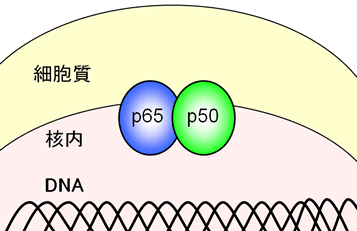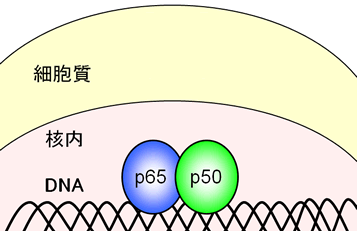
Since 10 years ago, it has been found that in many kinds of tumor and leukemia cells, NF-κB is found to be hyperactivated without a stimulus. It should be noted that hyperactivated NF-κB is especially detected in relatively high-malignant cancers and leukemia.
Estrogen and androgen are known to play an important role in the development of breast and prostate cancers, respectively, and an inhibition of those is the widely-used treatment of such cancers. Cancers that are vulnerable to a hormone treatment are relatively low in malignancy, while resistant ones, which overcome most of the treatments, are highly malignant. Those “tough” cancers, especially ones with resistance to the hormone treatment, are usually accompanied by a high level of NF-κB activity.
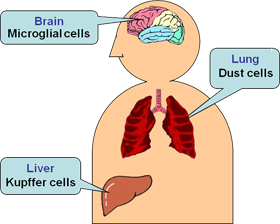
Macrophages usually work in a defense by engulfing and digesting cellular debris and by attacking and eliminating invaders like bacteria. When activated, they become enlarged, and so, they are called "giant phages". In our body, macrophages are found in almost all parts of organs, each of which is named differently.
Liver… “Kupffer cells”
Lung…“Dust cells”
Brain… “Microglia cells”
Exogenous stimuli may cause excess activation of those macrophages, leading to inflammation in many parts of a body. As a result, it turns out to be various diseases including rheumatism, arteriosclerosis, nephritis, and cachexia
NF-κB plays the central role in activating the macrophages in various diseases.
As you could see, high level of NF-κB activity deeply correlates to an initiation and development of intractable diseases involving cancers and inflammation. That is why inhibiting NF-κB is a reliable treatment for various malignant diseases.
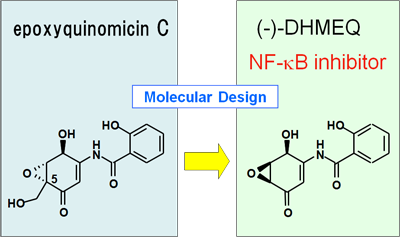
DHMEQ is a novel chemical substance that inhibits NF-κB. The name “DHMEQ” is an abbreviation of its structure, dehydroxymethylepoxyquinomicin, which we often called more shortly as “EQ”. In the experiments with animal models of cancer, leukemia, and rheumatism, DHMEQ exhibits an excellent inhibition effect, and thus it is expected to become a novel medical drug for various malignant diseases.

How was DHMEQ discovered? Just as a fashion designer speculates and draws an image of trendy styles of clothes, making an attempt to design clothes they have imagined, we are also able to design the molecular shape of drugs to supplement a specific characteristic.

Applying such a method called "molecular designing", we have developed DHMEQ.
There was a weak antibiotic called epoxyquinomycin. Because of its weak activity, it had never used as a medical drug. Ten years ago, we found that the structure of epoxyquinomycin was much similar to a known NF-κB inhibitor. Although epoxyquinomycin does not itself inhibit NF-κB activity, the newly designed drug, DHMEQ, remarkably inhibits an activity of NF-κB without a distinct side effect. That's the discovery story of DHMEQ.
DHMEQ can be synthesized in relatively a few steps. This is an advantage when it comes to talk about the clinical drug development. However, there still remain some challenges.
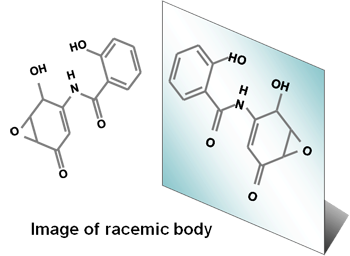
First, chemical synthesis only produces racemic DHMEQ, which consists of two mirror-imaged structures in one to one ratio. The substances that have the distinct, steric mirror-imaged structures are called "optically active", each denoted as (-) or (+). For example, if your left hand is (+) form, your right hand will be (-) form. In order to develop into a medical drug, either (-) or (+) needs to be strictly separated. As for DHMEQ, (-)-DHMEQ is ten times more effective than (+)-DHMEQ, and thus only (-)-DHMQ is the drug candidate.
Selective synthesis of (-) form is today's our challenge, but we are about to go through this challenge in cooperation with Keio University Faculty of Pharmaceutical Sciences, as well as with Signal Creation Inc.
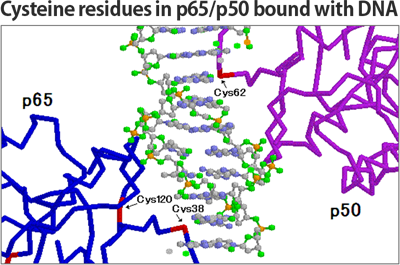
DHMEQ irreversibly binds to one of many Cysteine residues in a protein. Because NF-κB is a transcriptional factor, it binds to genomic DNA for transcription. Looking into a molecular model when NF-κB associates DNA, we find that DHMEQ selectively binds to the Cysteine residue of p65, cRel, RelB and p50, located at the proximal region of DNA. This shows that DHMEQ inhibits an activity of NF-κB by negating its binding to DNA.

Each component of NF-κB binds to a single DHMEQ molecule in an irreversible fashion to inhibit NF-κB activity. We have confirmed this by using a precise protein mass analysis called MALDI-TOF MS, perhaps more widely known as a method for which Dr. Tanaka Koichi has won the Noble prize. Addition of the same amount of DHMEQ to p65 results in the mass shift, which coincides with the exact molecular weight of DHMEQ. DHMEQ would be the only chemical among the other NF-κB inhibitors whose binding to NF-κB is proven by this prominent physic-chemical method. We believe that explains the specificity of DHMEQ to NF-κB. That is, DHMEQ is the specific NF-κB inhibitor that has been physico-chemically proven.
Thus, we have recently elucidated that DHMEQ, binding to a specific region of NF-κB, greatly inhibits its activity. It does not bind to any other proteins, ensuring a superior selectivity to the other developed NF-κB inhibitors. It also means to have a low toxicity to animals.
- 2012
- 2010-2011
- 2000-2009
- 1990-1999
- Bulk download
To view and print PDF documents, you need the Acrobat Reader program (now just called Adobe Reader). It is available as a no-charge download
from Adobe's web site.